Activating the innate immune system to revolutionize the treatment landscape for patients with CD30-positive lymphomas and EGFR- expressing solid tumors
While important advances have been made in the last few decades in the treatment of various hematologic and solid tumors, several remaining challenges require innovative medicines to address gaps in the treatment landscape. Affimed aims to overcome these challenges by developing novel innate cell engager (ICE®) molecules, which help restore the innate immune system in the fight against cancer. Innate immunity – the body’s first line of defense – eliminates infected or abnormal cells, including tumor cells, by activating natural killer (NK) cells, macrophages and other white blood cells. We believe our ICE® molecules could transform the field of immuno-oncology by giving patients back their innate ability to fight cancer.
Pursuing a first-in-class ICE® molecule to treat CD30-positive lymphomas
Among the CD30-positive cancers we are targeting is Hodgkin lymphoma, which originates in a type of white blood cell called a lymphocyte. Approximately 220,000 people in the U.S. are living with this hematologic cancer.(i) It occurs most commonly in people in their 20s and in those older than 55, and more often in males than in females. People with a family history of Hodgkin lymphoma and those with a weakened immune system may be at increased risk of developing the disease. (ii)
Existing therapies for Hodgkin lymphoma and other CD30-positive lymphomas may not work for everyone. For people suffering from CD30-positive Hodgkin lymphoma who relapse or are refractory to the standard of care, no therapies show durable efficacy or broad tolerability. For patients suffering from CD30-positive non-Hodgkin lymphoma subtypes, such as peripheral T-cell lymphoma (PTCL), a treatment gap remains, and some patients must resort to a clinical trial.
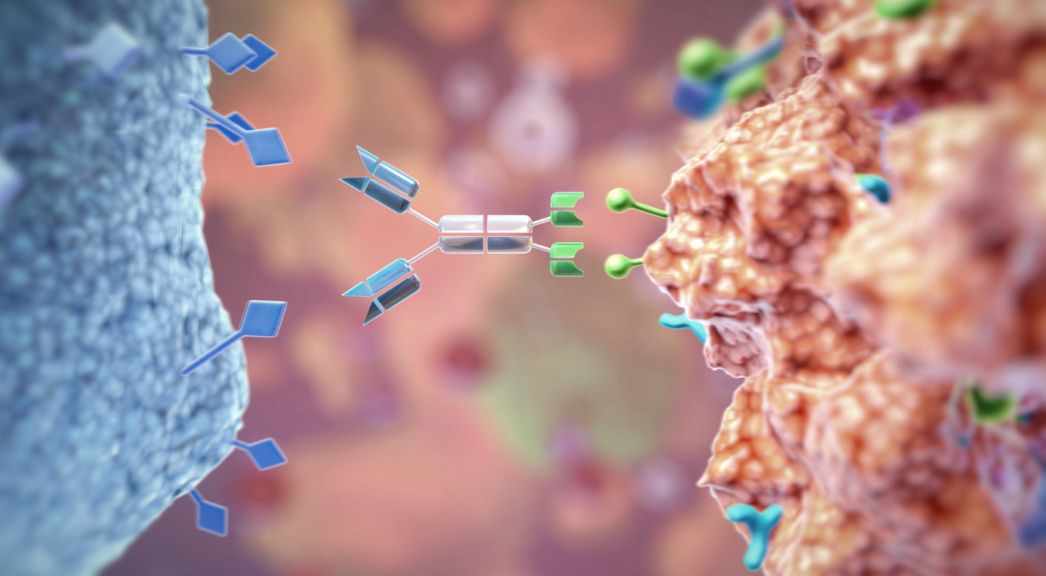
To address these limitations, Affimed is developing acimtamig (AFM13), a first-in-class ICE® molecule that represents a novel approach to activating innate immunity. Acimtamig binds to both CD16A, a receptor on innate cells, such as NK cells and macrophages, and CD30, a receptor found on several types of cancer cells. In doing so, it redirects innate immune cells to kill CD30-positive hematologic tumors. Administered in combination with natural killer cells, acimtamig may provide additional treatment options for patients suffering from CD30-positive lymphomas due to its unique properties.
AFM13 is currently being evaluated as a monotherapy for the treatment of Hodgkin lymphoma, PTCL, transformed mycosis fungoides and CD30-positive T-cell lymphoma. It is also being evaluated in combination with adoptive NK cells for the treatment of CD30-positive lymphomas, as well as in combination with other immuno-oncology therapies, such as checkpoint inhibitors.
To date, clinical trials evaluating acimtamig as a monotherapy and in combination with a check point inhibitor have shown signs of clinical efficacy. For patients with dysfunctional innate immune cells, which limits the body’s ability to fight cancer, early clinical studies indicate acimtamig in combination with adoptive NK cells showed impressive objective and complete response rates in heavily pretreated patients with relapsed/refractory Hodkin lymphoma and may provide therapeutic benefit for patients with other CD30-positive lymphomas.
Advancing the development of a novel ICE® molecule for EGFR-expressing solid tumors
Solid tumors that express a protein called epidermal growth factor receptor (EGFR) are difficult to treat, and people with EGFR-expressing tumors usually have a poor prognosis. Typically, EGFR helps cells grow, but, when mutated, it can lead to abnormal cell growth or cancer. EGFR is widely expressed on colorectal, lung, ovarian, gastric, breast and pancreatic cancers, which are among the most common and deadly types of cancer. Prognosis for those with EGFR-expressing solid tumors remains unfavorable because of intrinsic or acquired resistance to EGFR-targeted therapies, which can limit the use of these medicines to very specific patient populations. Additionally, some patients discontinue these therapies because of associated side effects.
To address the need for new therapeutics for EGFR-expressing solid tumors, we are developing AFM24, an ICE® molecule that targets EGFR-positive solid tumors. AFM24 has a distinct mechanism of action from current therapies. AFM24 activates the innate immune system by binding to both CD16A on innate cells and EGFR receptors on cancer cells, recruiting NK cells and macrophages to effectively and efficiently kill EGFR-expressing tumors.
We believe AFM24 has the potential to disrupt the treatment paradigm by becoming the first ICE® based therapy for patients with EGFR-expressing Non-Small Cell Lung Cancer. Because of its unique activation of the innate immune system, AFM24 could offer a safe and effective treatment option for patients regardless of mutations that hinder other therapies – and even work in patients whose tumors have become resistant to other therapies. AFM24 is currently being evaluated in combination with the checkpoint inhibitor atezolizumab.
AFM24 is currently being evaluated as a monotherapy for the treatment of multiple types of solid tumors, and additional trials are planned to explore AFM24 in combination with adoptive NK cells, as well as a PD-L1 inhibitor, to treat multiple types of EGFR-positive tumors. AFM24 has demonstrated a favorable safety profile in preclinical studies, and it has the potential to overcome the safety concerns associated with existing therapies.
Targeting CD123 with Affimed’s ICE® molecule AFM28 to benefit patients with Acute Myeloid Leukemia
In addition to acimtamig and AFM24, we are developing our ICE® molecule AFM28 which targets CD123 and CD16A. A phase 1 study with patients with Acute Myeloid Leukemia is ongoing and a combination approach with allogeneic NK cells is being evaluated.
i https://seer.cancer.gov/statfacts/html/hodg.html ii https://www.cancer.org/cancer/hodgkin-lymphoma/causes-risks-prevention/risk-factors.html