Earnings - April 28, 2020
Heidelberg, Germany, April 28, 2020 – Affimed N.V. (Nasdaq: AFMD), a clinical stage biopharmaceutical company committed to giving patients back their innate ability to fight cancer, today reported financial results for the year ended December 31, 2019 and provided an update on clinical and corporate progress.
“Our clinical trials currently remain active, and we are working closely with our participating physicians and clinical sites to minimize the impact on further progress as they focus on addressing the evolving COVID-19 global health crisis,” said Dr. Adi Hoess, Affimed’s CEO. “During these unprecedented and challenging times, our top priority continues to be supporting our employees, patients and our healthcare partners, while ensuring the continuity of our operations.”
Affimed remains committed to continuing its development programs but acknowledges the impact from the evolving COVID-19 pandemic on clinical studies, including potential delays in patient enrollment. The company plans to update expected timing of milestones for its clinical studies after there is more clarity on the duration and magnitude of the impact from the COVID-19 pandemic.
AFM13 (CD30/CD16A)
AFM24 (EGFR/CD16A)
COVID-19 has significantly impacted the global healthcare system, including the conduct of clinical trials as medical institutions prioritize the treatment of those afflicted with COVID-19. Affimed has taken several important actions to balance the commitment to treat cancer patients and to mitigate potential health and safety risks posed by the COVID-19 pandemic, while maintaining continuity of its operations and preserving financial flexibility for the future.
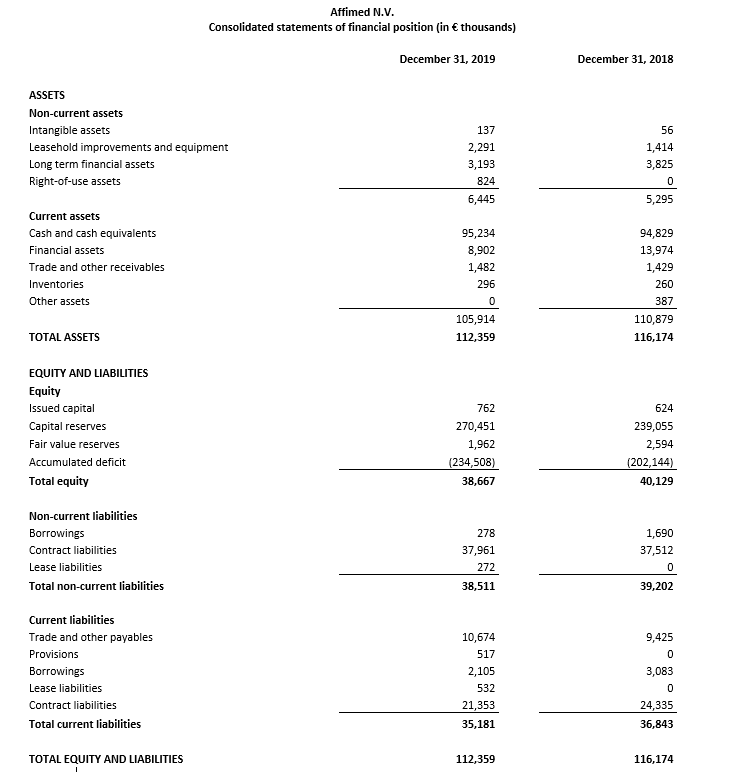
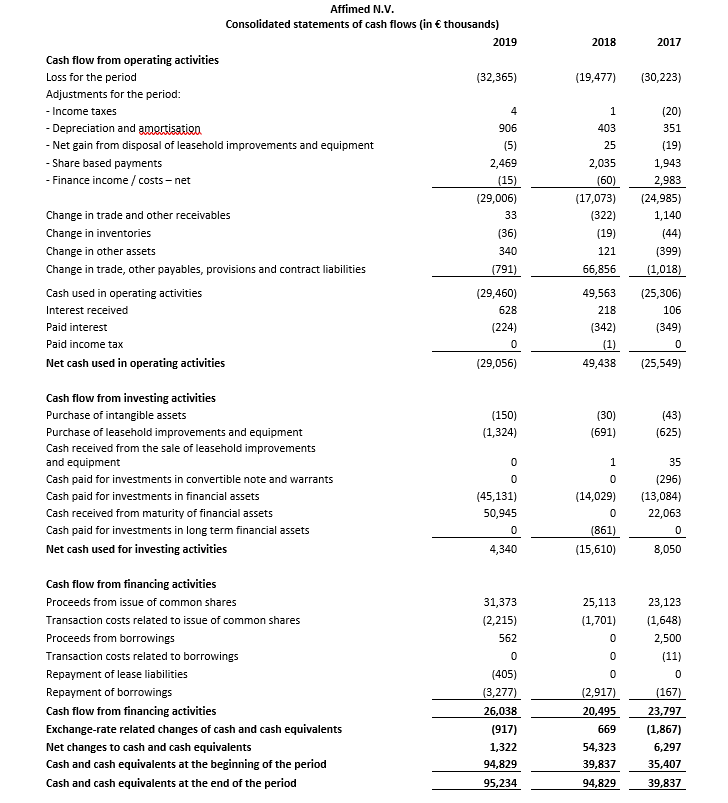
Cash, cash equivalents and current financial assets totaled €104.1 million as of December 31, 2019 compared to €108.8 million as of December 31, 2018. During the fourth quarter of 2019, the company raised €29.5 million in net proceeds, after deducting underwriting discounts and commissions and estimated offering expenses, from a completed public offering. Based on its current operating plan and assumptions, Affimed anticipates that its cash, cash equivalents and current financial assets as of December 31, 2019 will support operations at least into the first half of 2022.
Net cash used in operating activities was €29.1 million for the twelve months ended December 31, 2019, compared to net cash from operating activities of €49.4 million for the twelve months ended December 31, 2018. The net cash from operating activities in 2018 includes an initial upfront payment and committed funding of €83.2 million ($96.0 million) from the strategic collaboration Affimed entered into with Genentech Inc. in August 2018.
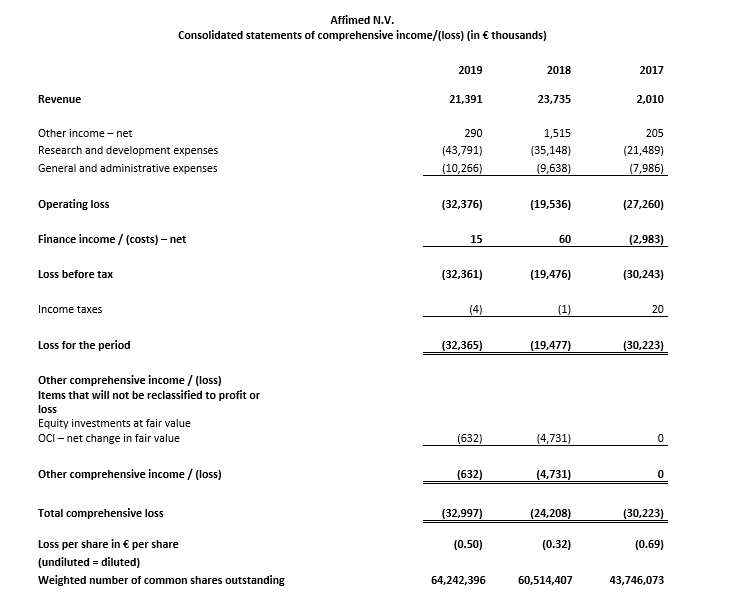
Total revenue was €21.4 million for the year ended December 31, 2019 compared to €23.7 million for the year ended December 31, 2018. Revenue in both years is attributable primarily to the recognition of revenue from the Genentech collaboration in the respective years.
Research and development (R&D) expenses were €43.8 million for the year ended December 31, 2019, compared to €35.1 million for the year ended December 31, 2018. The increase was primarily related to higher expenses for startup activities for the AFM13 registration-directed study in pTCL, manufacturing activities for AFM13 clinical study material, and early stage development and discovery activities.
General and administrative (G&A) expenses were €10.3 million for the year ended December 31, 2019, compared to €9.6 million for the year ended December 31, 2018. In 2019, G&A expenses were primarily related to personnel expenses and legal, consulting and audit costs.
Net loss was €32.4 million, or €0.50 per common share, for the year ended December 31, 2019, compared to a net loss of €19.5 million, or €0.32 per common share, for the year ended December 31, 2018.
Additional information regarding these results is included in the notes to the consolidated financial statements as of December 31, 2019 and “Item 5. Operating and Financial Review and Prospects,” which will be included in Affimed’s Annual Report on Form 20-F as filed with the U.S. Securities and Exchange Commission (SEC).
Affimed prepares and reports the consolidated financial statements and financial information in accordance with IFRS as issued by the International Accounting Standards Board. None of the financial statements were prepared in accordance with Generally Accepted Accounting Principles in the United States. Affimed maintains its books and records in Euro.
Affimed will host a conference call and webcast today, Tuesday, April 28, 2020 at 8:30 a.m. Eastern time to discuss the company’s 2019 financial results and recent corporate developments. The conference call will be available via phone and webcast. To access the call, please dial +1 (877) 870-9135 for U.S. callers, or +44 (0) 2071 928338 for international callers, and reference passcode 7978047 approximately 15 minutes prior to the call. A live audio webcast of the conference call will be available in the “Webcasts” section on the “Investors” page of the Affimed website at https://www.affimed.com/investors/webcasts_cp/, and a replay of the webcast will be accessible at the same link for 30 days following the call.
Affimed (Nasdaq: AFMD) is a clinical-stage immuno-oncology company committed to giving patients back their innate ability to fight cancer. Affimed’s fit-for-purpose ROCK® platform allows innate cell engagers to be designed for specific patient populations. The company is developing single and combination therapies to treat hematologic and solid tumors. The company is currently enrolling patients into a registration-directed study of AFM13 for CD30-positive relapsed/refractory peripheral T cell lymphoma and into a Phase 1/2a dose escalation/expansion study of AFM24 for the treatment of advanced EGFR-expressing solid tumors. For more information, please visit www.affimed.com.
This press release contains forward-looking statements. All statements other than statements of historical fact are forward-looking statements, which are often indicated by terms such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “goal,” “intend,” “look forward to,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would” and similar expressions. Forward-looking statements appear in a number of places throughout this release and include statements regarding our intentions, beliefs, projections, outlook, analyses and current expectations concerning, among other things, the potential of AFM24, the value of our ROCK® platform, our ongoing and planned preclinical development and clinical trials, our collaborations and development of our products in combination with other therapies, the timing of and our ability to make regulatory filings and obtain and maintain regulatory approvals for our product candidates, our intellectual property position, our collaboration activities, our ability to develop commercial functions, clinical trial data, our results of operations, cash needs, financial condition, liquidity, prospects, future transactions, growth and strategies, the industry in which we operate, the trends that may affect the industry or us, impacts of the COVID-19 pandemic, the benefits to Affimed of orphan drug designation and the risks, uncertainties and other factors described under the heading “Risk Factors” in Affimed’s filings with the SEC. Given these risks, uncertainties and other factors, you should not place undue reliance on these forward-looking statements, and we assume no obligation to update these forward-looking statements, even if new information becomes available in the future.
Gregory Gin, Head of Investor Relations
E-Mail: IR@affimed.com