ICE® (innate cell engager) molecules bring together the innate immune cell (NK cell or macrophage) and tumor cell by binding to the CD16A receptor on innate immune cells and a specific receptor on the tumor cells. Once this bridge is established, the immune cell is activated to release factors that directly kill the tumor cell.
In contrast with other antibody-based therapeutics, ICE® (innate cell engager) molecules generated from the ROCK® (Redirected Optimized Cell Killing) platform avoid competition with the body’s own circulating serum IgG by binding efficiently and stably to a unique epitope on the CD16A receptor on NK cells and macrophages with high affinity and avidity.1,2

Our ICE® (innate cell engager) molecules restore dysregulated NK cells, and increase cytotoxic activity
of healthy donor–derived NK cells 3,4
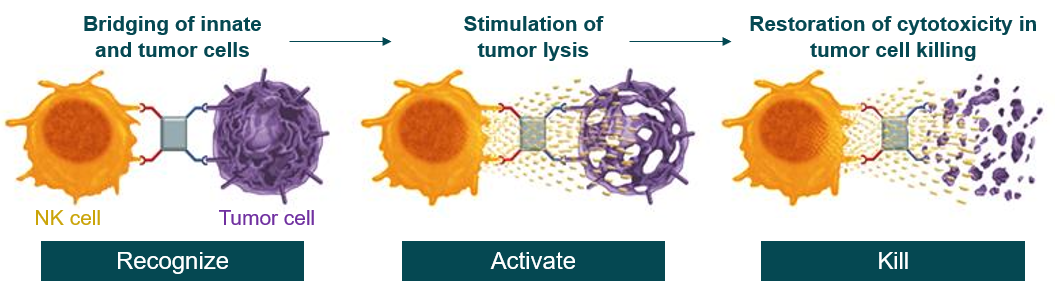
Innate immune cell reactivation through ICE® (innate cell engager) molecules enables tumor recognition and initiates an immune response. When the NK cell and tumor cell are brought together, the innate immune cell releases perforins to create pores in the tumor cell membrane through which granzymes enter the tumor cell, triggering apoptosis and resulting in tumor cell death.
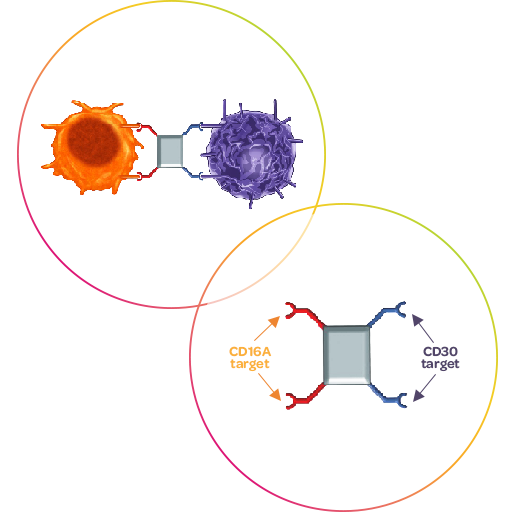
Acimtamig is Affimed’s first-in-class ICE® (innate cell engager) molecule targeting CD30 on cancer cells and CD16A on innate immune cells. It has shown antitumor activity as monotherapy in a number of clinical trials leading to meaningful results for patients with CD30-positive lymphomas.4, 5, 6
When used in combination with an anti–PD-1 antibody in Hodgkin lymphoma, acimtamig demonstrated promising efficacy, including an ORR of 88% at the highest treatment dose, as well as a CR of 46% (per independent assessment).7 Both ORR and CR rates were higher in combination with acimtamig than with the anti-PD-1 antibody alone. 7
When combined with fresh cord blood-derived natural killer (NK) cells, acimtamig yielded unprecedented results in a phase 1/2a study in heavily pre-treated patients with Hodgkin lymphoma and Non-Hodgkin lymphoma. There were 32 patients with Hodgkin lymphoma, treated at the recommended Phase 2 dose, who achieved a 97% ORR rate and a CR rate of 78%. The median event-free survival (EFS) of the same cohort was 9.8 months with 84% of the patients alive at 12 months, and median duration of response (DoR) was 8.8 months. The treatment demonstrated a good safety and tolerability profile.8 Affimed’s new phase 2 study, LuminICE-203, builds on these data and investigates acimtamig in combination with alloNK® an off-the-shelf, allogeneic NK cell from Artiva Biotherapeutics, in patients with r/r classical Hodgkin lymphoma and an exploratory arm in r/r/ PTCL (NCT05883449).
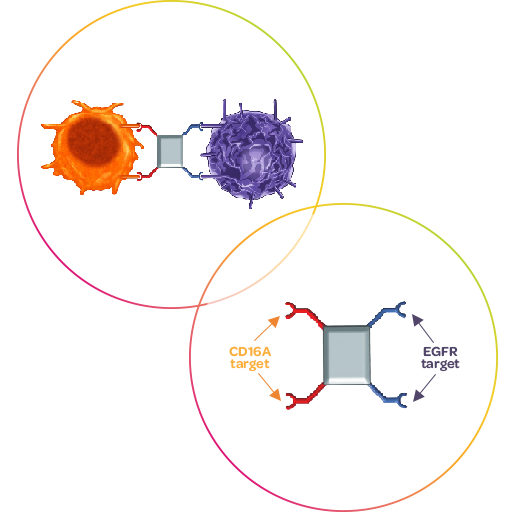
AFM24 is an EGFR-directed ICE® molecule, which exhibits a distinctive mechanism of action that engages innate immune cells by recruiting NK cells and macrophages to the site of the tumor for mediating an effective and efficient anti-tumor response.9 It thereby does not rely on the EGFR signaling pathway, but uses EGFR as a docking site only, which suggests that AFM24 can be used in all EGFR-expressing tumors, including tumors that have become resistant to signaling inhibition. 9
Based on the encouraging signals of activity and its well-managed safety profile seen in monotherapy (NCT04259450),10,11 Affimed is focusing development of AFM24 as combination therapy with atezolizumab in patients with Non-Small Cell Lung Cancer (NSCLC) with encouraging initial results (NCT05109442).12
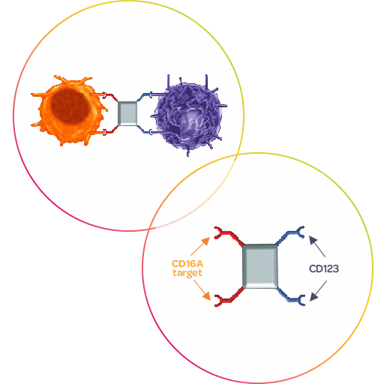
AFM28 is an ICE® that targets CD123 on leukemic cells and CD16A on NK cells, thereby mediating NK cell induced antibody-dependent cellular cytotoxicity (ADCC). It is being developed for patients with acute myeloid leukemia (AML) who are in need of new therapeutic options, because they are either primary refractory to or have relapsed after treatment. AML treatment failures are attributed to the persistence of leukemic stem cells (LSCs), a subpopulation of AML cells with self-renewal and leukemia-initiating capacities, which have been shown to be highly refractory to conventional antileukemic treatments and are believed to be the fundamental cause of drug resistance and AML relapse. Therefore, effective depletion of leukemic blasts as well as eradication of residual LSCs is crucial for long-term remission. AFM28 was shown to efficiently induce lysis of CD123-positive leukemic cell lines and to control the tumor outgrowth in an in vivo mouse model of AML. Additionally, AFM28 mediated strong NK cell-dependent depletion of both CD123-positive leukemic blasts and LSCs from AML patient samples thereby exhibiting a capacity to induce long-term remission, and preventing disease relapse after conventional treatment or allogeneic stem cell transplant in patients with AML .13
A first-in-human Phase 1 clinical study has been initiated in 2023 and development in combination with allogeneic NK cell therapy is being explored.
ADCC=antibody-dependent cellular cytotoxicity; cbNK=cord blood–derived natural killer cell; CD=cluster of differentiation; CR=complete response; EGFR=epidermal growth factor receptor; I-O=immuno-oncology; IgG=immunoglobulin G; MOA=mechanism of action; NK=natural killer; ORR=objective response rate; PD-1=programmed cell death protein 1.
References: 1. Ellwanger K, Reusch U, Fucek I, et al. Redirected optimized cell killing (ROCK®): A highly versatile multispecific fit-for-purpose antibody platform for engaging innate immunity. MAbs. 2019;11(5):899-918. 2. Wingert S, Reusch U, Baez A, et al. CD16A-specific tetravalent bispecific immuno-engagers potently induce antibody-dependent cellular phagocytosis (ADCP) by macrophages. Poster presented at: American Society of Hematology (ASH) Annual Meeting; December 1-4, 2018; San Diego, CA. 3. Zhao X, Rajasekaran N, Reusch U, et al. In vitro and in vivo characterization of CD19/CD3 Tandab AFM11 and CD19/CD16A Tandab AFM12 targeting NHL. Poster presented at: American Society of Hematology (ASH) Annual Meeting; December 5-8, 2015; Orlando, FL. 4. Rothe A, Sasse S, Topp MS, et al. A phase 1 study of the bispecific anti-CD30/CD16A antibody construct AFM13 in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2015;125(26):4024-4031. 5. Kim WS, Shortt J, Zinzani PL, et al. REDIRECT: A Phase 2 study of AFM13 in patients with CD30-positive relapsed or refractory (R/R) peripheral T cell lymphoma (PTCL). AACR 2023. CT024. https://www.aacr.org/wp-content/uploads/2023/05/AACR2023_LBA-Trials_AbstractsEbook.pdf 6. Sawas A, Chen P, Vlad G, et al. Clinical and biological evaluation of the novel CD30/CD16A tetravalent bispecific antibody (AFM13) in relapsed or refractory CD30-positive lymphoma with cutaneous presentation: a biomarker Phase Ib/IIa study (NCT03192202). Poster presented at: American Society of Hematology (ASH) Annual Meeting; December 1-4, 2018; San Diego, CA. Final data available here: History of Changes for Study: NCT03192202 (clinicaltrials.gov). 7. Bartlett NL, Herrera AF, Domingo-Domenech E, et al. A phase 1b study of AFM13 in combination with pembrolizumab in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2020;136(21):2401-2409. 8. Affimed Press Release 11 December 2023. 9. Wingert S, Reusch U, Knackmuss S et al. Preclinical evaluation of AFM24, a novel CD16A-specific innate immune cell engager targeting EGFR-positive tumors. mAbs. 2021;13(1): e1950264. 10. El-Khoueiry AB, Rivas D, Lee S-H et al. Leveraging innate immunity with AFM24, a novel CD16A and epidermal growth factor receptor (EGFR) bispecific innate cell engager: Interim results for the non-small cell lung cancer (NSCLC) cohort. Poster presented at: American Society of Clinical Oncology (ASCO) Annual Congress; June 9-13, 2023. Poster 375. Abstract: J Clin Oncol 41, 2023 (suppl 16; abstr 2533). 11. Hintzen G, Wingert S, Emig M, et al. Targeting Epidermal Growth Factor Receptor (EGFR)-Expressing Solid Tumors With AFM24, A Novel CD16A Bispecific Innate Cell Engager: Comprehensive Correlative Science Findings From A Phase 1 Study. Poster presented at: Society for Immunotherapy of Cancer’s (SITC) 37th Annual Meeting; November 8-12, 2022. Poster 729. 12. Affimed Press Release 11 December 2023. 13. Schmitt N, Siegler J-J, Wagner L, et al. The Novel Bispecific Innate Cell Engager (ICE®) AFM28 Efficiently Directs Allogeneic NK Cells to CD123-positive leukemic cells. Poster presented at: American Society of Hematology (ASH) Annual Meeting; December 10-13, 2022; New Orleans, Louisiana.