Earnings - March 27, 2019
AFM13: Encouraging data supports initiation of monotherapy registration-directed study in first half of 2019; Combination study with cord blood-derived natural killer cells planned to initiate at MD Anderson; Clinical data updates from monotherapy study and combination study with Keytruda® (pembrolizumab) expected in 2019
AFM24: IND submission planned for mid-2019; Plans to initiate first-in-human study in second half of 2019
Heidelberg, Germany, March 27, 2019 – Affimed N.V. (Nasdaq: AFMD), a clinical stage biopharmaceutical company committed to giving patients back their innate ability to fight cancer, today provided an update on recent operational progress and reported financial results for the year ended December 31, 2018.
“2018 was a year in which Affimed made substantial progress. We achieved tremendous clinical and corporate milestones that further demonstrated our innate immunity expertise and the potential for therapeutics based on our novel CD16A-targeting innate cell engager approach to treat cancer,” said Dr. Adi Hoess, Affimed’s CEO. “With our current financial resources, including funds received from Genentech under our collaboration, we believe we are well-positioned to achieve additional milestones in 2019 and beyond, including advancing our lead development candidate, AFM13, into a market registration-directed study and entering the clinic with AFM24, our second innate cell engager and a potential treatment for multiple solid tumor malignancies.”
CD16A innate cell engager programs
AFM13 (CD30/CD16A)
AFM24 (EGFR/CD16A)
T cell engager programs
AFM11 (CD19/CD3)
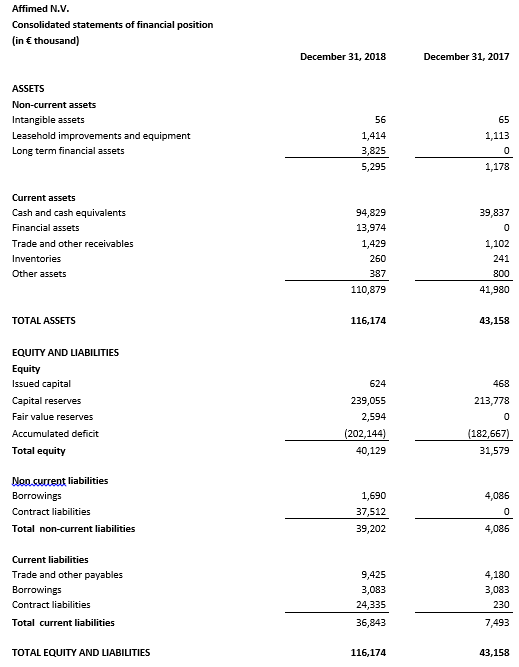
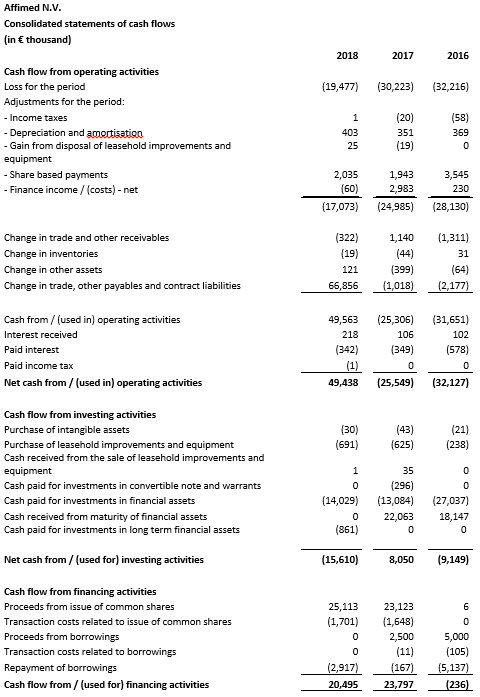
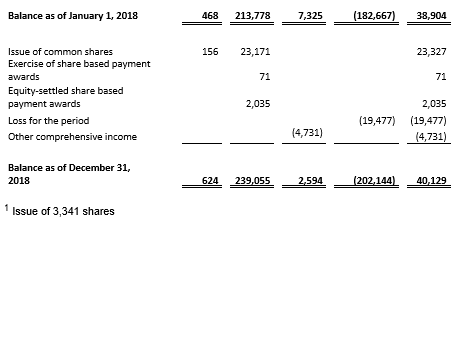
Cash, cash equivalents and current financial assets totaled €108.8 million as of December 31, 2018 compared to €39.8 million as of December 31, 2017. Affimed anticipates that its cash, cash equivalents and current financial assets as of December 31, 2018 will enable the Company to fund its operations, including clinical development and early development activities, into 2021 assuming all of the Company’s programs advance as currently contemplated.
Net cash from operating activities was €49.4 million for the twelve months ended December 31, 2018 compared to net cash used in operating activities of €25.5 million for the twelve months ended December 31, 2017. The amount in 2018 includes an initial upfront payment and committed funding of €83.2 million from the Genentech collaboration.
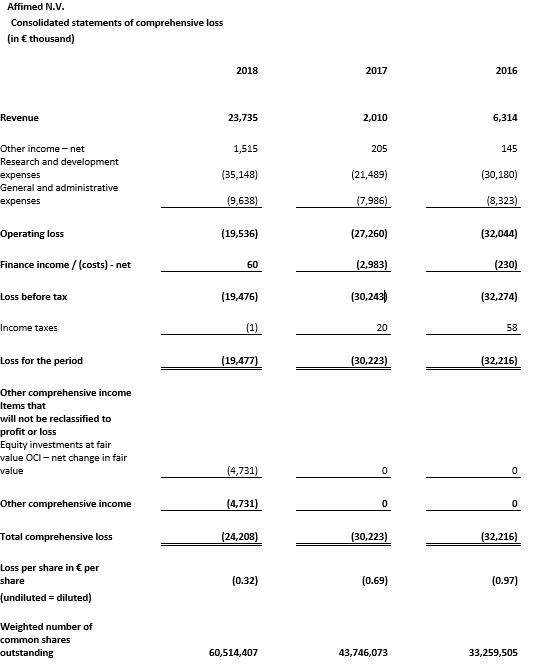
Affimed recognized €21.8 million as revenue from the Genentech collaboration in 2018 and €61.4 million under contract liabilities, which will be recognized as revenue in subsequent periods. Total revenue was €23.7 million for the year ended December 31, 2018 compared to €2.0 million for the year ended December 31, 2017.
Research and development (R&D) expenses were €35.1 million for the year ended December 31, 2018 compared to €21.5 million for the year ended December 31, 2017. The increase was related to higher expenses for AFM13 and AFM11 clinical development activities, as well as early stage development and discovery activities.
General and administrative (G&A) expenses were €9.6 million for the year ended December 31, 2018, compared to €8.0 million for the year ended December 31, 2017. This increase was primarily related to higher legal and consulting expenses.
Net loss was €19.5 million, or €0.32 per common share, for the year ended December 31, 2018, compared to a net loss of €30.2 million, or €0.69 per common share, for the year ended December 31, 2017. The decrease in net loss was primarily related to significantly increased revenue, partially offset by higher spending on R&D and G&A expenses.
Additional information regarding these results is included in the notes to the consolidated financial statements as of December 31, 2018 and “Item 5. Operating and Financial Review and Prospects,” which will be included in Affimed’s Annual Report on Form 20-F as filed with the U.S. Securities and Exchange Commission (SEC).
Affimed prepares and reports the consolidated financial statements and financial information in accordance with International Financial Reporting Standards (IFRS) as issued by the International Accounting Standards Board (IASB). None of the financial statements were prepared in accordance with Generally Accepted Accounting Principles (GAAP) in the United States. Affimed maintains its books and records in Euro.
Affimed will host a conference call and webcast today, Wednesday, March 27, 2019 at 8:30 a.m. Eastern time to discuss the Company’s financial results and recent corporate developments. To access the call, please dial +1 (631) 510-7495 for U.S. callers, or +44 (0) 2071 928000 for international callers, and reference conference ID 5559486 approximately 15 minutes prior to the call. An audio webcast of the conference call can be accessed in the “Webcasts” section on the “Investors” page of the Affimed website at https://www.affimed.com/investors/webcasts/. A replay of the webcast will be available on Affimed’s website shortly after the conclusion of the call and will be archived for 30 days following the call.
Affimed (Nasdaq: AFMD) is a clinical stage biopharmaceutical company committed to giving back patients back their innate ability to fight cancer. Affimed’s fit-for-purpose ROCK® platform allows innate immune engagers to be designed for specific patient populations. The Company is developing single and combination therapies to treat cancers. For more information, please visit www.affimed.com.
This press release contains forward-looking statements. All statements other than statements of historical fact are forward-looking statements, which are often indicated by terms such as "anticipate," "believe," "could," "estimate," "expect," "goal," "intend," "look forward to", "may," "plan," "potential," "predict," "project," "should," "will," "would" and similar expressions. Forward-looking statements appear in a number of places throughout this release and include statements regarding our intentions, beliefs, projections, outlook, analyses and current expectations concerning, among other things, the value of our ROCK® platform, our ongoing and planned preclinical development and clinical trials, our collaborations and development of our products in combination with other therapies, the timing of and our ability to make regulatory filings and obtain and maintain regulatory approvals for our product candidates our intellectual property position, our collaboration activities, our ability to develop commercial functions, expectations regarding clinical trial data, our results of operations, cash needs, financial condition, liquidity, prospects, future transactions, growth and strategies, the industry in which we operate, the trends that may affect the industry or us and the risks uncertainties and other factors described under the heading “Risk Factors” in Affimed’s filings with the Securities and Exchange Commission. Given these risks, uncertainties and other factors, you should not place undue reliance on these forward-looking statements, and we assume no obligation to update these forward-looking statements, even if new information becomes available in the future.
Gregory Gin,
Head of Investor Relations
E-Mail: IR@affimed.com
Anca Alexandru,
Head of Communications, EU IR
E-Mail: media@affimed.com